History
In the late 1950s, Prof. Dr. med. Carl Erich Alken had the idea to make it easier to perform catheterisation. His approach involved no longer wetting urological instruments, such as catheters and cystoscopes, with a viscous, "lubricating" substance, but rather to fill the urethra itself with a lubricant. Instillation of the lubricant was intended to expand the folds of the star-shaped urethra and keep them open during the procedure, enabling urological instruments to be inserted into the urethra with almost no resistance. This was simultaneously intended to considerably reduce the risk of injury.
Prof. Alken perfected his product idea by deciding to include a combination of active substances with disinfecting and local anaesthetic properties in the lubricant. This was intended to considerably reduce the occurrence of urinary tract infections and the spread of bacteria and decrease the risk of injury because the patient would experience no pain during the procedure and remain relaxed.
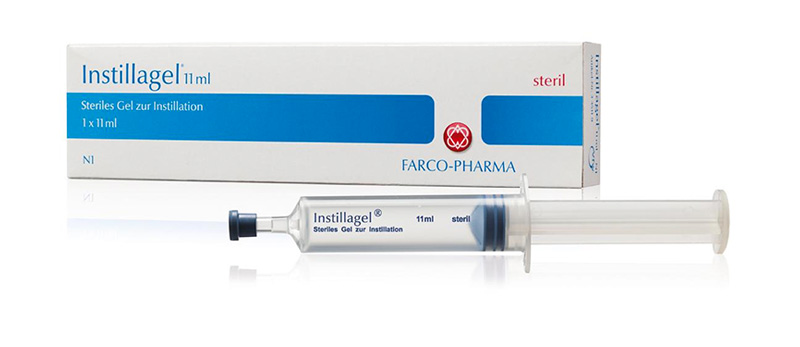
In order to implement his idea, Prof. Alken needed a pharmaceutical partner and found it in 1964 at FARCO-PHARMA GmbH in the Hessian city of Giessen. Small-scale, initial production of the lubricant with the name Instillagel® that was supplied with an application syringe began at the pharmacy of Mr. Reinhard, the company co-founder.
Demand for Instillagel® rose very rapidly within Germany and several customers from abroad also showed interest in the lubricant. As a result, initial shipments of the lubricant to Austria began as early as 1970. The company owners hadn`t expected such a huge demand. Due to their limited production facilities, the two found themselves unable to ensure the supply of Instillagel® at the required volumes. As a result, they decided to sell the company.
In 1971, FARCO-PHARMA GmbH moved its headquarters to Cologne, Germany. The pharmaceutical formula for Instillagel® was reengineered, systematic sterile production began, and the modified lubricant was launched domestically.
With the objective of making this urological milestone accessible to the international market as well, sales of Instillagel® were expanded to several European countries, including Switzerland, the Netherlands, Belgium, Luxembourg, and the UK.
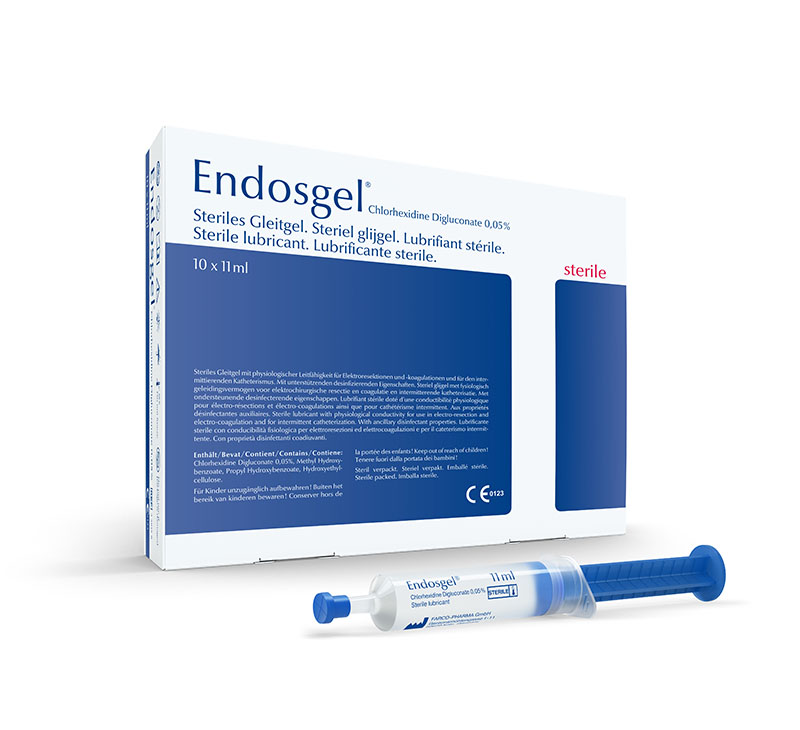
In addition to the Instillagel® medicinal product, FARCO-PHARMA continued to expand its product portfolio over time. In 1978, as second lubricant was launched called Endosgel®, ready-to-use syringe with physiological electrical conductivity and auxiliary disinfecting properties.
The exportation of FARCO-PHARMA products also expanded and in 1990 Hong Kong became the first overseas market for Instillagel®. Over the ensuing years, the number of export markets continued to grow and FARCO-PHARMA products are now sold in many countries, including Ireland, Sweden, Italy, France, Portugal, United Arab Emirates and Indonesia.
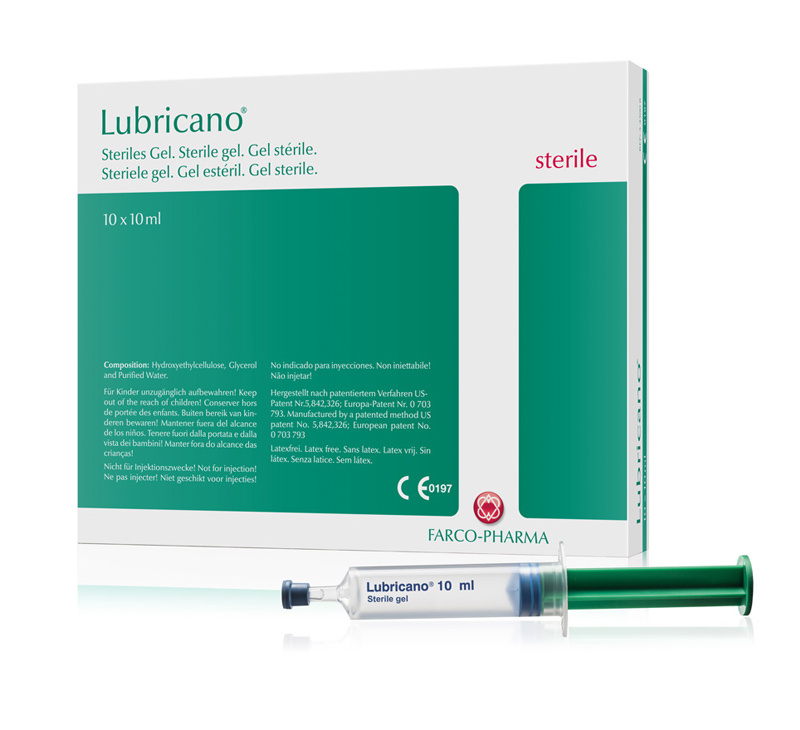
In 2001, a third lubricant was launched named Lubricano®. As a lubricant with no active ingredient, Lubricano® is exceptionally well tolerated and ideally suited for application in patients with allergies and intolerances to certain active substances.
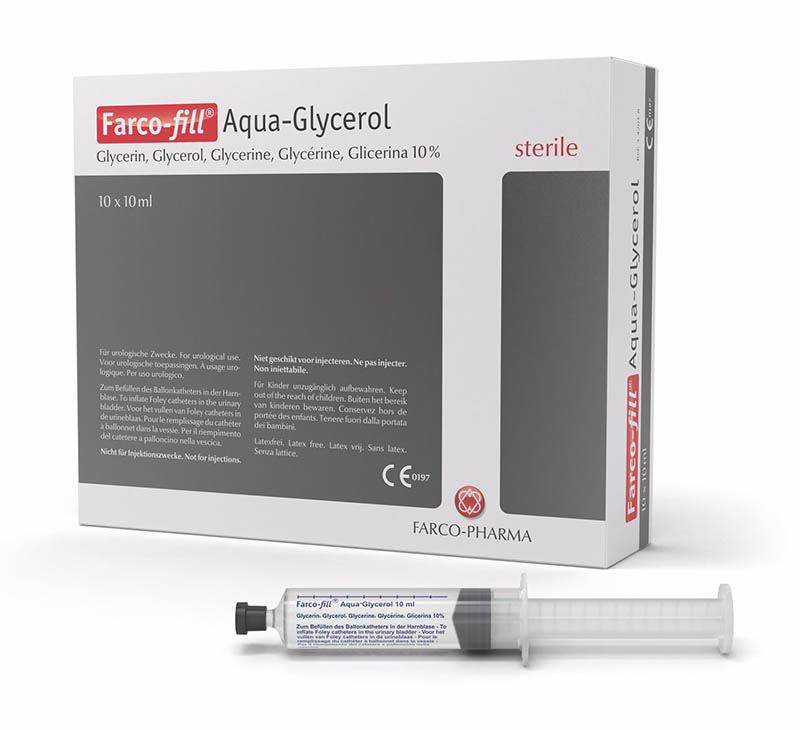
In 2001, in addition to the lubricants, FARCO-PHARMA also launched its first sterile solution for catheter inflation named Farco-fill® Aqua-Glycerol for indwelling catheters.
FARCO-PHARMA exports continued to grow and Canada became the first market for FARCO-PHARMA products in North America in 2006. Egypt, the first market in Africa, followed three years later and in 2011 Australia, a whole other continent, became the next market for FARCO-PHARMA products.
Since 2012, FARCO-PHARMA has been selling its products in Columbia, the first South American market for the company's products.
In addition to lubricants, the German specialist and market leader for urological lubricants has since launched three sterile solutions for catheter inflation as medical devices for filling balloon catheters. The sterile solutions for catheter inflation Aquatouch® (launched in 2015) and the Farco-fill® Aqua-Glycerol launched in 2001 are purified water-based or water-glycerol solutions for indwelling catheters. Farco-fill® Protect was launched in 2013.
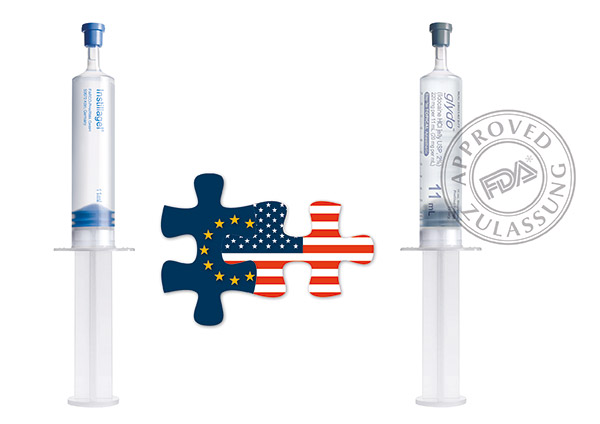
Another milestone in FARCO-PHARMA's history was achieved in 2014 with the successful approval of the GLYDO® catheter lubricant for the U.S. market by the U.S. Food and Drug Administration (FDA).
Today, FARCO-PHARMA products are sold on every continent and the company has access to a global network of 44 sales partners.
-
Austria becomes the first export market for Instillagel®
-
Hong Kong becomes the first overseas export market
-
Canada becomes the first market for FARCO-PHARMA products in North America
-
Egypt becomes the first market for FARCO-PHARMA products in Africa
-
Australia and New Zealand become markets for FARCO-PHARMA products
-
Columbia becomes the first market for FARCO-PHARMA products in South America
FARCO-PHARMA products now sold on every continent